CDISC SDTM Models
- Home
- CDISC SDTM Models
- Background on CDISC SDTM Standards
- Advantages of SDTM
- Latest CDISC SDTM Standards and Implementation Guides
- Demonstrate SDTM Mapping
- SDTM Compliance
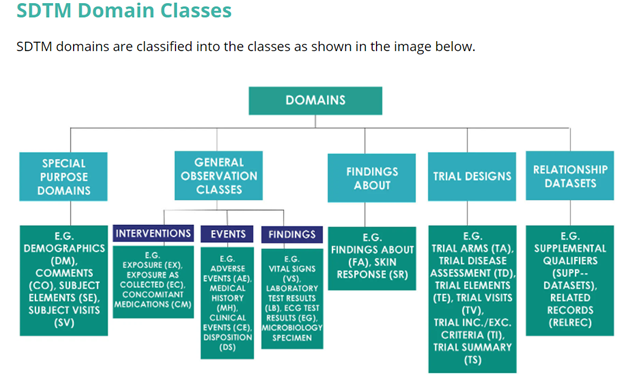
The CDISC standard domain models (SDTMIG 3.2)
Special-Purpose Domains:
- Comments (CO)
- Demographics (DM)
- Subject Elements (SE)
- Subject Visits (SV)
Interventions General Observation Class:
-
Concomitant Medications (CM)
- Exposure as Collected (EC)
- Exposure (EX)
- Substance Use (SU)
- Procedures (PR)
Events General Observation Class:
- Adverse Events (AE)
- Clinical Events (CE)
- Disposition (DS)
- Protocol Deviations (DV)
- Medical History (MH)
- Healthcare Encounters (HO)
Findings General Observation Class:
- Drug Accountability (DA)
- Death Details (DD)
- ECG Test Results (EG)
- Inclusion/Exclusion Criterion Not Met (IE)
- Immunogenicity Specimen Assessments (IS)
- Laboratory Test Results (LB)
- Microbiology Specimen (MB)
- Microscopic Findings (MI)
- Morphology (MO)
- Microbiology Susceptibility Test (MS)
- PK Concentrations (PC)
- PK Parameters (PP)
- Physical Examination (PE)
- Questionnaires (QS)
- Reproductive System Findings (RP)
- Disease Response (RS)
- Subject Characteristics (SC)
- Subject Status (SS)
- Tumor Identification (TU)
- Tumor Results (TR)
- Vital Signs (VS)
Findings About :
- Findings About Events or Interventions (FA)
- Skin Response (SR)
Trial Design Domains:
- Trial Arms (TA)
- Trial Disease Assessment (TD)
- Trial Elements (TE)
- Trial Visits (TV)
- Trial Inclusion/Exclusion Criteria (TI)
- Trial Summary (TS)
Special-Purpose Relationship Datasets:
- Qualifiers - SUPPQUAL
- Relate Records - RELREC

