CDISC Standards Basic
CDISC Foundational Standards are the basis of the complete suite of standards, supporting clinical and non-clinical research processes from end to end. Foundational Standards focus on the core principles for defining data standards and include models, domains and specifications for data representation.
For researchers – clear and simple data management at all stages of clinical research meaning that breakthroughs happen faster, and study results are more easily shared and understood by the community.
For pharma companies – simple and fast submission and review process as CDISC standards are required by FDA, PMDA, and EMA.
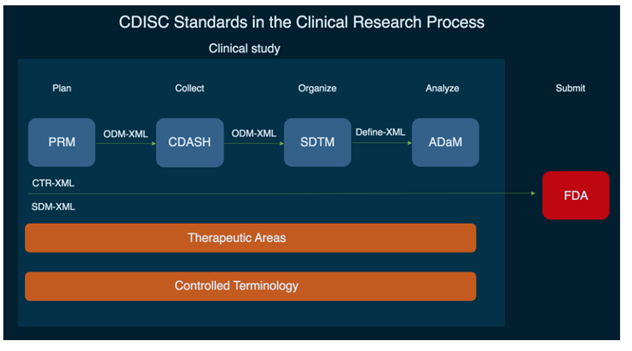
Basically, CDISC standards are divided into four groups:
Foundational or data content standards (SEND, PRM, CDASH, SDTM, ADaM). These are the basis standards for a lot of other CDISC standards that support data collection, management, analysis, and reporting at all stages of the clinical study process.
Data exchange standards (SDM-XML, ODM-XML, Define-XML, CTR-XML). CDISC uses a typical XML standard for data exchange, supported by industry-related specifications.
Therapeutic areas. These standards specify foundational standards for different disease areas. For example, here’s a user guide explaining how to collect data and conduct trials relevant to COVID-19.
Controlled terminology and glossary. This is a dataset containing a list of terms that should be used with CDISC standards, ensuring that data is collected and recorded consistently.
Data exchange standards
The fundamental standard for data exchange in the clinical trial field is ODM or Operational Data Model. ODM framework uses XML to exchange data across the whole healthcare ecosystem: electronic data capture (EDC), electronic health records (EHR), data collection, tabulation, analysis, and archival. This data includes clinical report forms (CRFs) and datasets in a SDTM format.
As all CDISC standards for data exchange, it has an XML specification, which enables its transformation into PDF, Word, HTML, and other formats very easily. This is the main standard for moving data and metadata between different stages of clinical data management. All CDISC data exchange standards are actually extensions of ODM.
